Table of Contents
Precision Where Lives Depend on Performance
Medical technology demands perfection. Every circuit, connection, and component must meet the highest standards of quality and reliability because human lives depend on it.
At Foxtronics EMS, we understand that PCB assembly manufacturing for the medical industry requires more than technical skill. It requires process discipline, full traceability, and compliance with rigorous standards such as ISO 13485. We deliver electronics solutions that meet these expectations, from early design support to final system assembly and testing.
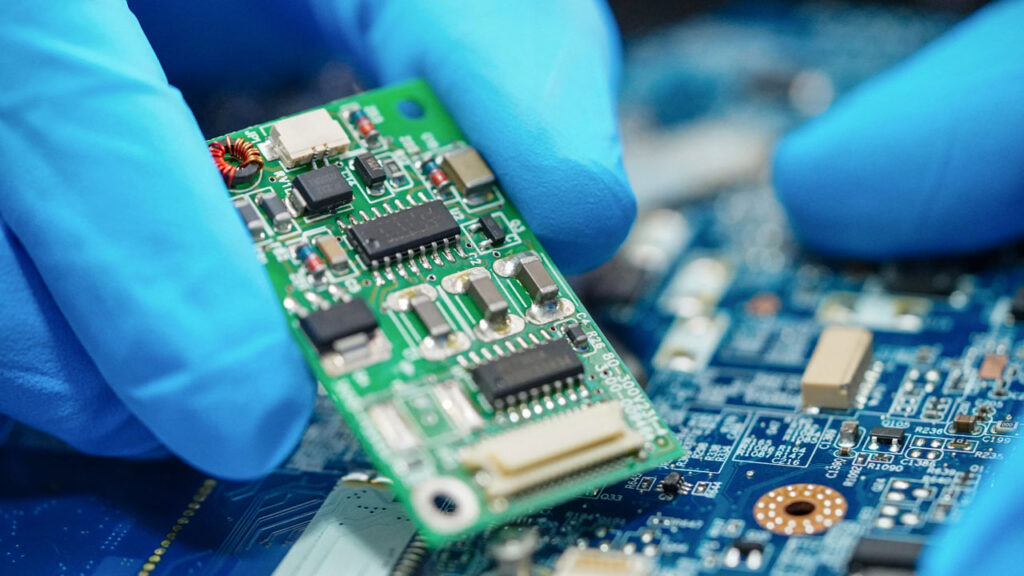
The Unique Demands of Medical Electronics Manufacturing
Medical electronics combine innovation with strict regulatory oversight. Devices used in diagnostics, monitoring, and treatment must function flawlessly in environments where failure is not an option.
Foxtronics supports these requirements through:
- Controlled manufacturing environments
- Detailed process documentation
- Complete component traceability
- Testing protocols aligned with customer and regulatory standards
By maintaining this level of control, we help ensure that every medical product performs safely, consistently, and predictably.
ISO 13485 Certified Quality Systems
Foxtronics operates under a quality management system aligned with ISO 13485, the international standard for medical device manufacturing. This certification defines how we manage risk, maintain documentation, and verify process control throughout the production cycle.
Our system includes:
- Design and process validation
- Documented inspection and test records
- Material traceability from supplier to shipment
- Corrective and preventive action management
These controls ensure that every process step is measurable, auditable, and repeatable, building trust in every product we deliver.
Design for Manufacturability and Test
Many medical devices are complex, combining miniaturized electronics with sensors, displays, and communication modules. Foxtronics provides Design for Manufacturability (DFM) and Design for Test (DFT) support to help customers create reliable, cost-effective designs.
By collaborating early in the design stage, our engineers help optimize:
- PCB layout for solderability and cleanliness
- Component selection for long-term availability
- Test access for electrical and functional verification
- Mechanical integration for enclosure assembly
This partnership reduces time to market while ensuring compliance and reliability from the first prototype to full production.
Controlled Manufacturing and Process Discipline
Medical electronics manufacturing demands consistency across every production lot. Foxtronics EMS employs detailed work instructions, barcode tracking, and in-process verification to guarantee repeatability.
Our facility includes:
- ESD-controlled production areas
- Automated SMT and through-hole assembly lines
- In circuit and functional test capabilities
- Precision cleaning and inspection stations
Each PCB assembly is verified to ensure that it meets customer specifications and documented quality standards before shipment.
Traceability and Documentation
Traceability is the backbone of compliance in the medical sector. Foxtronics provides complete traceability from raw materials to final assemblies through digital record keeping and serialized tracking.
Our documentation includes:
- Lot and serial number traceability for every component
- Automated record storage for inspection and test data
- Full process history for each product
- Material certifications and supplier documentation
This ensures that every unit can be traced back to its origin, allowing full accountability and rapid response if any field issue arises.
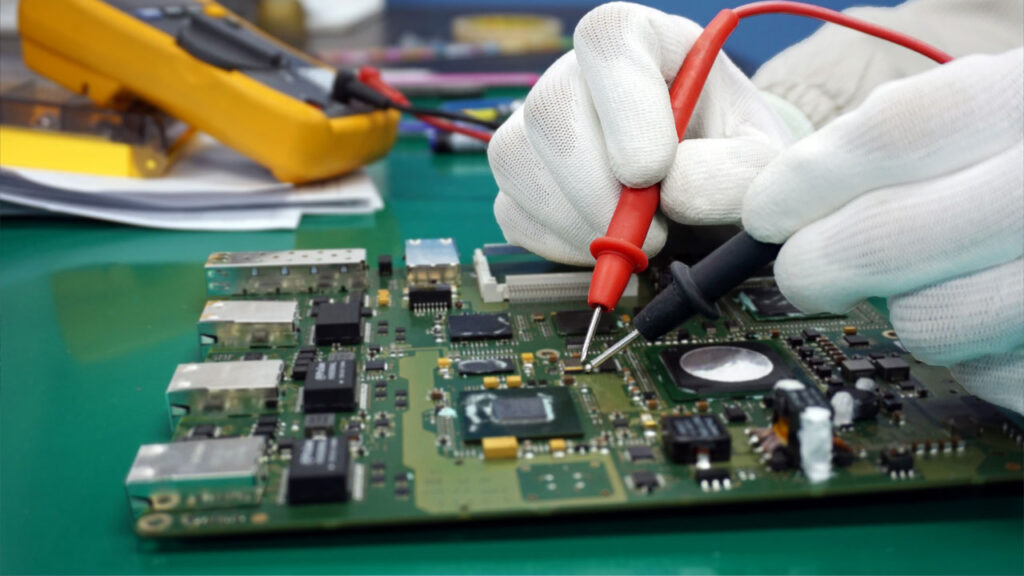
Testing and Validation for Patient Safety
Foxtronics EMS conducts electrical, functional, and environmental testing on all medical PCB assemblies to confirm compliance and performance. We validate every board under conditions that replicate its intended use.
Testing may include:
- Functional verification under simulated load
- Burn-in and temperature cycling
- Signal integrity and isolation testing
- Visual and AOI inspection for solder and placement accuracy
By validating performance before shipment, we ensure that each device operates reliably in the hands of healthcare professionals and patients.
Supporting Innovation in Medical Technology
The medical industry is advancing rapidly, with growth in wearable devices, diagnostic imaging, portable monitors, and telemedicine systems. Foxtronics EMS partners with innovators across these sectors, providing the manufacturing expertise needed to move from concept to commercial production.
We specialize in supporting both established OEMs and emerging startups that require flexibility, precision, and strict compliance. Our team provides the agility of a collaborative partner with the discipline of a regulated manufacturer.
Conclusion: Reliability You Can Trust in Critical Care
At Foxtronics EMS, we treat every medical product with the care and precision it deserves. Our processes, people, and quality systems are designed to deliver electronics that meet the highest industry standards and patient safety requirements.
Through discipline, documentation, and collaboration, we help medical innovators bring life-improving technologies to market, reliably and responsibly.
If your medical device program demands precision, compliance, and reliability, Foxtronics EMS is ready to help. Contact our team to learn how our medical electronics manufacturing expertise can support your next innovation.
