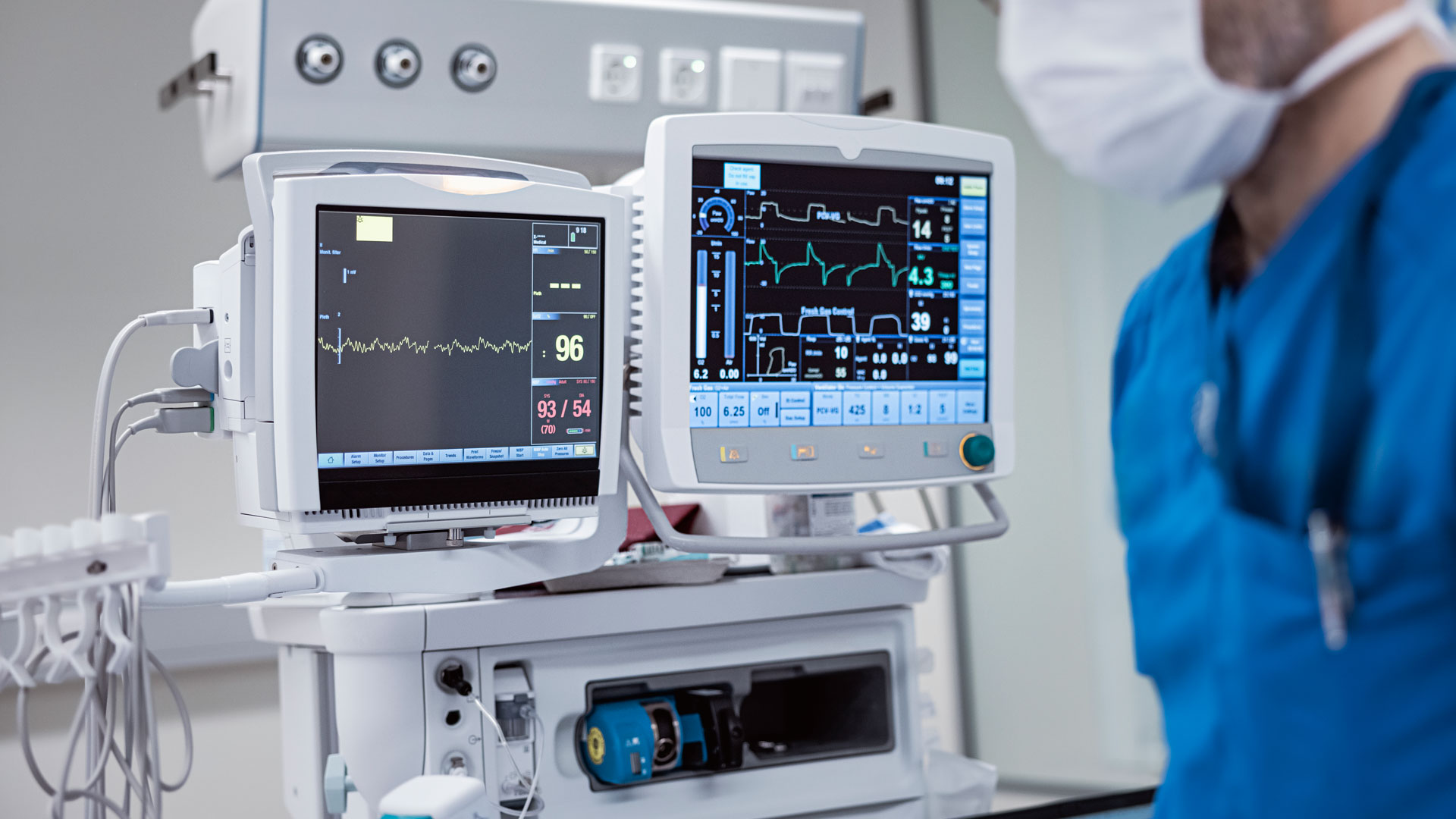
PCB Assembly for Medical & Life Sciences
ISO 13485-certified PCB assembly and electronics manufacturing for medical and life-science devices, with full documentation, traceability, and consistency from prototype to production.
In medical and life-science applications, reliability is non-negotiable. Every board, connection, and component plays a role in patient care, from surgical instruments and diagnostic analyzers to wearable devices and imaging systems. Foxtronics EMS—a group of trusted medical PCB assembly manufacturers in the US—offers end-to-end medical PCB assembly services. We deliver precision, process control, and accountability at every stage of PCBA manufacturing, ensuring your medical devices perform flawlessly when it matters most.
We collaborate with medical OEMs and device innovators from design through production, aligning engineering intent with manufacturability and regulatory requirements. Our teams follow strict ISO 13485 and IPC Class 3 standards, with clean, ESD-controlled PCB assembly environments, traceable supply chains, and audit-ready documentation.
From rapid prototyping and design validation through high-mix, low-volume production and full-scale medical and healthcare electronics manufacturing, Foxtronics EMS delivers the consistency and compliance that the global medical and life sciences industry demands. Our disciplined approach to medical PCB assembly services reduces risk, accelerates product validation, and provides complete transparency, giving OEMs the confidence to focus on innovation while we ensure flawless execution.

Electronics Manufacturing for the Medical Industry demands deep manufacturing experience and the discipline required by ISO 13485, IPC Class 3, and FDA-aligned standards. Unlike commercial electronics, medical systems must maintain precise performance under all conditions: heat, vibration, sterilization, and continuous use. A minor PCB assembly error can lead to device failure, which in healthcare could mean the difference between success and risk.
That’s why Foxtronics EMS integrates design for manufacturability (DFM) expertise, rigorous process validation, and complete documentation into every medical device build to reduce redesigns and accelerate time-to-market. Each medical device PCB assembly undergoes comprehensive testing and validation, including functional, in-circuit, and reliability testing protocols that mirror real-world clinical use conditions. Test data is logged under serialized identifiers for traceability and long-term compliance.
Our controlled environments, clean PCB assembly processes, and stringent quality systems ensure every medical PCBA build meets FDA and CE regulatory standards. The goal isn’t just meeting specifications; it’s ensuring patient safety, product consistency, and complete accountability at every stage of medical PCB assembly production.
Medical and life-science OEMs trust Foxtronics EMS for PCB assemblies that meet or exceed specifications. We support leading manufacturers with precise, compliant, and traceable builds from early prototyping through full-scale production.
Every medical PCB assembly is built, inspected, and documented under our ISO 13485-certified quality system, ensuring compliance with global regulatory standards with precision and care. Clean, compliant, and ready to perform when trust matters most.
We deliver high‑density, mixed‑technology PCB assemblies for diagnostic, imaging, monitoring, and wearable medical devices. Our expertise spans surface‑mount (SMT) for compact, high‑performance designs and through‑hole assembly for durable, mechanically robust connections. Each build is produced under ISO 13485 standards with full optical inspection to ensure reliability.
End-to-end mechanical and system integration for finished medical devices, including cabling, enclosure assembly, regulatory labeling, and calibration. Each product receives full functional verification and safety inspection before release.
We implement functional, in-circuit, and reliability testing to IEC and FDA standards. All data is archived digitally to maintain full traceability for audits and device history records.
Our design and engineering expertise provides early-stage design reviews that identify potential risks and cost-saving opportunities. Our engineers work alongside your team to streamline NPI transitions, eliminate validation delays, and accelerate regulatory approvals.
Our reliable Supply Chain Management system ensures validated suppliers, component lot traceability, and electronic documentation to meet regulatory standards, with complete DHR (Device History Record) archives kept audit-ready.
Choosing the right EMS partner in the medical & life sciences industry means choosing someone who understands what’s at stake. Foxtronics EMS is that partner. We combine rigorous process control with responsive engineering collaboration to help OEMs meet quality, compliance, and delivery goals with confidence.
Our commitment extends beyond PCB assembly. We offer aftermarket services and lifecycle management, including device repair, refurbishment, field support, and product upgrades throughout the device’s operational lifetime. In an industry where reliability is measured in lives improved or saved, our role is to ensure the electronics inside every medical and healthcare device perform exactly as intended, every single time.
We uphold strict quality, safety, and compliance standards backed by advanced inspections, thorough testing, and globally recognized certifications to ensure product reliability and performance.
Every product undergoes rigorous in-line inspections, functional testing, and quality control processes at every stage of production to guarantee consistency and precision.
Foxtronics EMS maintains internationally recognized certifications that reflect our dedication to quality management and regulatory compliance across multiple industries:
With decades of experience and industry certifications, Foxtronics EMS supports leading innovators in demanding sectors, delivering reliable PCB assembly for mission-critical products. Our certified quality systems, advanced manufacturing, and engineering expertise ensure we meet the unique technical requirements of every industry we serve.
Foxtronics EMS combines precision, reliability, and scalability to deliver high-performance PCB assembly solutions. With decades of experience, advanced facilities, and engineering expertise, we add value across every stage of your product’s lifecycle.
With over 116 years of combined experience, Foxtronics EMS serves industries such as aerospace, medical, and industrial. Our AS9100, ISO, and ITAR certifications ensure compliance with the highest quality and regulatory standards, allowing us to meet the demands of mission-critical applications.
From initial design to final delivery, we offer complete lifecycle support. Our in-house engineering team provides DFM and early design consultation to optimize manufacturability, reduce risk, and accelerate time-to-market—ensuring a smooth transition from concept to scalable production.
Foxtronics EMS provides fully managed turnkey solutions, including component sourcing, SMT and through-hole assembly, box builds, and testing. This approach simplifies supply chains, reduces vendor dependency, and ensures consistent quality at every stage.
Our state-of-the-art facilities are designed to handle complex PCB assemblies with exceptional precision and reliability. Automated systems, high-speed SMT lines, and rigorous process controls ensure consistent quality and scalability, supporting the most intricate and demanding production requirements.
Quality assurance is built into every step of our process. We utilize AOI, X-ray inspection, in-circuit, and functional testing to detect defects early and ensure long-term reliability. Our inspection protocols align with IPC and AS9100 industry standards.
With flexible production and streamlined workflows, we offer rapid prototyping and efficient scaling for diverse production needs. Whether for low-volume prototypes or high-volume runs, our responsive team ensures fast, reliable delivery without sacrificing quality.
Our facilities are purpose-built for precision and flexibility, supporting everything from quick-turn prototypes to high-volume PCBA production. With dedicated areas for assembly, inspection, and testing, every process runs under controlled workflows to ensure consistent quality at scale.
Find quick answers about our ISO 13485-certified medical PCB assembly services, compliance standards, and how we ensure reliability and patient safety in every medical device build.
We support a broad range of programs across diagnostic, therapeutic, and life-science applications — from analyzers and imaging modules to patient-monitoring devices, wearable health platforms, and lab-automation assemblies. Our experience spans both electromechanical systems and sensor-based PCBs requiring high precision and clean handling.
Yes. Our facilities operate under ISO 13485 certification and follow FDA 21 CFR 820-aligned quality systems. That means documented risk management, controlled process validation, and device history records that withstand regulatory audits.
Every medical PCB assembly is serialized and digitally tracked from component receipt through final shipment. Process parameters, inspection data, and test results are automatically logged, allowing complete traceability and fast retrieval during audits or CAPA reviews.
Absolutely. We run NPI and quick-turn prototype builds within the same controlled environment as production. Early engagement lets our engineers validate the layout, materials, and test coverage, so you can enter the verification and validation phases with confidence.
Functional and in-circuit testing, boundary-scan, and burn-in are standard options. We also perform temperature, humidity, and vibration testing that replicate field and clinical environments, with data logging integrated into the device’s documentation record. For deeper insight into how we manage heat and environmental stress in PCB assemblies, explore our thermal management techniques.
While we don’t act as a regulatory consultant, our documentation and process transparency make audit preparation far easier. We routinely support customer teams during ISO, CE, and FDA audits with production records, traceability reports, and process validations.